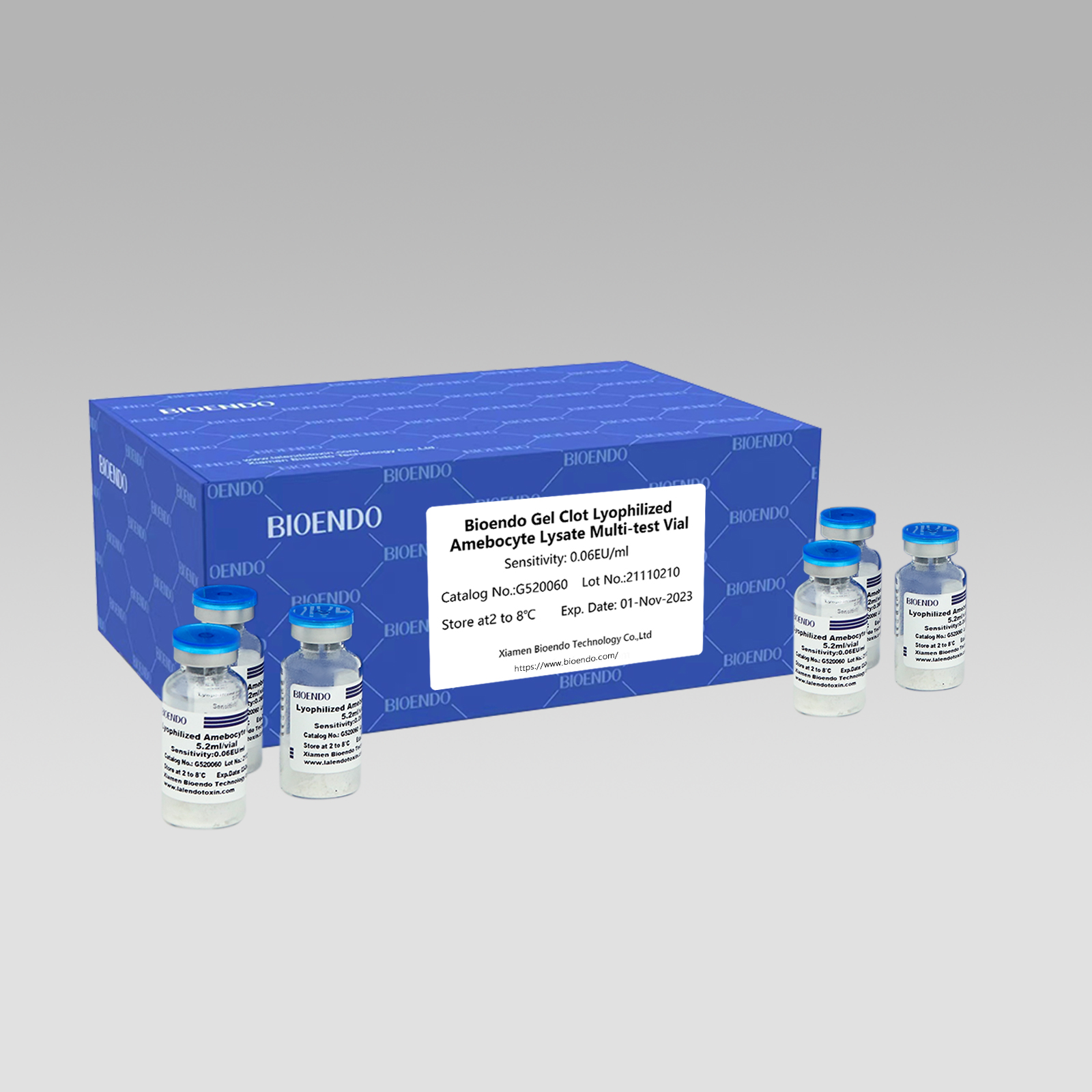
Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52
Bioendo G52 series inonyanya kushandiswa mukuedza kushanda kwebhakitiriya endotoxin bvunzosenzira yeBioassay.
1. Product Information
Gel Clot nzira Lyophilized Amebocyte Lysate Multi-test Vial ndiyo Lyophilized Amebocyte Lysate reagent iyo inosarudza uye kushandisa nzira yegel clot kuona endotoxin kana pyrogen.
Senzira yakapararira, gel-clot bvunzo ye endotoxin iri nyore uye haidi chakananga uye chinodhura chiridzwa.Bioendo inopa Gel Clot Lyophilized Amebocyte Lysate - LAL reagent mu 5.2ml per vial.
2. Product Parameters
Sensitivity range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Product Application
End-chigadzirwa endotoxin (pyrogen) qualification, mvura yejekiseniendotoxin assay, raw materialkuongorora endotoxinkana endotoxin level yekutarisa panguva yekugadzira maitiro emakambani emishonga kana vagadziri vemidziyo yekurapa.
Cherechedza:
Lyophilized Amebocyte Lysate (LAL reagent) inogadzirwa neBioendo inogadzirwa kubva ku lysate yeamebocytes (masero machena eropa) kubva kugaka rebhiza.
Iyi yakasarudzika reagent yave chishandiso chakakosha mumaindasitiri emishonga uye yezvokurapa ekuona kwebhakitiriya endotoxins.Amebocytes ehoveshoe crab ane chinhu chinonzi Lyophilized Amebocyte Lysate, chinopindirana nebhakitiriya endotoxins nekugadzira gel-like clot.Kuita uku ndiyo hwaro hwekuyedzwa kweLAL, hunoshandiswa kuve nechokwadi chekuchengetedzwa kwemidziyo yekurapa, mishonga, uye zvimwe zvigadzirwa zvinosangana nemuviri wemunhu.
Iko kushandiswa kweLAL reagent kwakashandura maitiro ekuwanikwa kwe endotoxinmune zvekurapa pane iyo Rabit test assay.Kunzwa kwayo kusingaenzaniswi uye kujeka kunoita kuti ive chinhu chakakosha mukutonga kwemhando uye vimbiso yekuchengetedza yemishonga, biologics, uye zvigadzirwa zvekurapa.Iyo LAL bvunzo inzira yekukurumidza uye yakavimbika yekuwanikwa kwe endotoxin, zvichipa mhinduro mukati memaminitsi makumi matanhatu.Kushanda uku kunobvumira kukurumidza uye kwakaringana sarudzo maererano nekuburitswa kwezvigadzirwa, pakupedzisira kunosimudzira kuchengetedzeka kwese uye kushanda kwekurapa nemidziyo.
Bioendo's Lyophilized Amebocyte Lysate (LAL reagent) inogadzirwa pasi peyakasimba mhando zviyero kuti ive nechokwadi chekushanda kwayo uye kuvimbika.Kambani yakatsaurirwa kushandisa maitiro akasimba mukukohwewa kwegakanje remabhiza kuti kuderedze chero kukanganisa kwakaipa pahuwandu hwavo.Nekuisa pamberi pekugara zvakanaka kwezvisikwa izvi, Bioendo inovimbisa kuenderera mberi kweiyo yakakosha sosi yekugadzirwa kweLAL reagents.Pamusoro pezvo, kuenderera mberi kwekutsvagisa nekusimudzira kuedza kwakanangana nekuvandudza mashandiro uye kuwanda kweLAL test endotoxin, vachienderera mberi nekufambisira mberi kwekushandisa kwavo mumaindasitiri ekurapa nemishonga.
Gel clot nziraLAL kuongorora, yakadzokororwa lysate reagent inowana angangoita makumi mashanu bvunzo pa vial:
| Catalog Number | Sensitivity (EU/ml kana IU/ml) | ml/mudziyo | Miedzo/Vial | Vials/Pack |
| G520030 | 0.03 | 5.2 | 50 | 10 |
| G520060 | 0.06 | 5.2 | 50 | 10 |
| G520125 | 0.125 | 5.2 | 50 | 10 |
| G520250 | 0.25 | 5.2 | 50 | 10 |
| G520500 | 0.5 | 5.2 | 50 | 10 |
Chigadzirwa chimiro:
Iyo Lyophilized Amebocyte Lysate - LAL reagent senitivity uye iyo Kudzora Standard Endotoxin potency inoedzwa kurwisa USP Reference Standard Endotoxin.Iyo Lyophilized Amebocyte reagent kits inouya nemirairo yechigadzirwa, Chitupa cheAnalysis, MSDS.
Ndeupi musiyano uripo pakati peBioendo single test vial uye multiple test vial?
● Muedzo mumwechete: gadzirisa imwe chetelimulus lysate bvunzokana kudanwalimulus amebocyteneBET mvura mugirazi vial kana girazi ampoule.
● Multi-test: gadzirisa lysate reagent nemvura yeBET, uye wobva wawedzera yakaratidza huwandu hwe lysate reagent inotevera COA kune reaction chubhu kana tsime ndiro yekushandisa.Iko hakuna mutsauko mumuenzaniso pre-processing maitiro;maererano nehuwandu hwekuedzwa hunoshandiswa, saizi yemuenzaniso inoshandiswa pabvunzo imwe chete yakakura kupfuura saizi yemuenzaniso inoshandiswa pabvunzo dzakawanda.
Nei iyo gel clot assay kit G52 yakakosha kune akawanda masampuli huwandu?
1. Multi test LAL reagent yekuonekwa kwe endotoxin mumashandisirwo emasampunzi akawanda 'LAL assay operation maitiro.
2. G52 series yeGel clot endotoxin assay multi test vial haidiwi sophisticated microplate reader.MuLAL assay maitiro ayo ekuisa incubation nemvura yekugezesa kana yakaoma kupisa incubator iri nyore mudziyo.
3. High end quality ye endotoxin free tube (<0.005EU/ml) uye High quality yepyrogen free tips (<0.005EU/ml) seyakavimbiswa kushandiswa kuti uve nechokwadi chemugumisiro wakarurama.
4. Kusarudza Bioendo single LAL test vial kana multi LAL test vial nehuwandu hwemasampuli, chinangwa chiriLAL bvunzo yepyrogenskuonekwa.
Zvigadzirwa zvinoenderana muendotoxin test assay:
Mvura yeBacterial Endotoxins Test (BET), Recommend TRW50 kana TRW100
Endotoxin yemahara girazi chubhu ( dilution chubhu), Recommend T1310018 uye T107540
Pyrogen yemahara matipi, Recommend PT25096 kana PT100096
Pipettor, kurudzira PSB0220
Test Tube Rack
Incubation Instrument (Kugezesa Mvura kana Dry Heat Incubator), kukurudzira Bioendo Dry Heat Incubator TAL-M2 maburi makumi matanhatu imwe modular.
Vortex Mixter, kurudzira VXH.
Kudzora Standard Endotoxin, CSE10V.