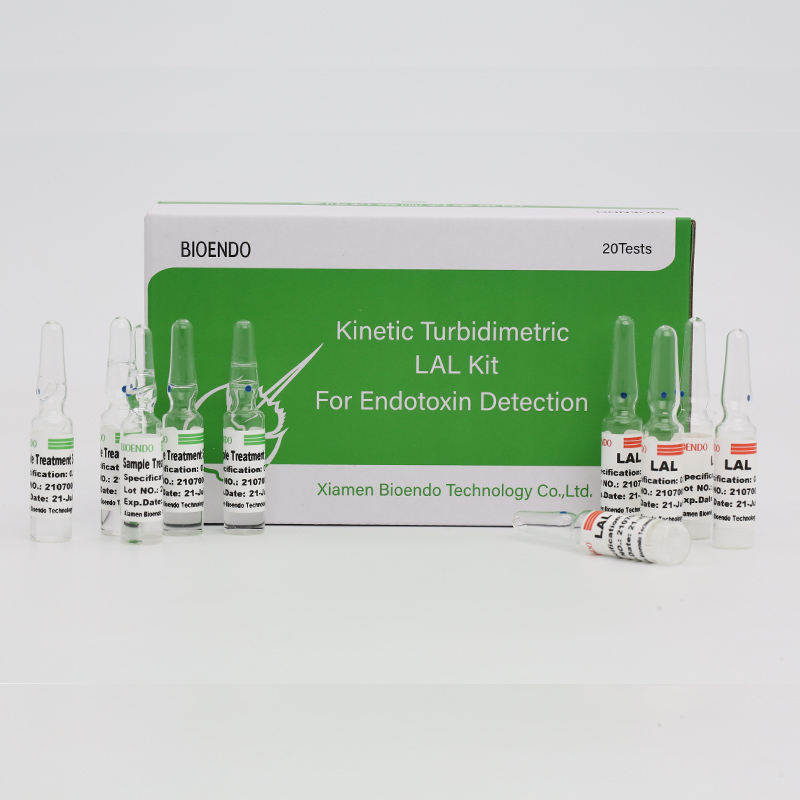
Endotoxin Assay Kit yePlasma Yemunhu
Endotoxin Assay KityePlasma yeMunhu
1. Product Information
CFDA yakabviswaClinical diagnostic Endotoxin assay kitinokwana endotoxin level inhuman plasma.Endotoxin chikamu chikuru chesero madziro eGram Negative mabhakitiriya uye ndiyo inonyanya kukosha microbial murevereri we sepsis.Yakakwira mwero we endotoxin inowanzokonzera fivha, shanduko muchena masero eropa uye, mune dzimwe nguva, kuvhunduka kwemoyo.Inobva pachinhu Cpathway mu limulus Polyphemus (horseshoe crab blood) bvunzo.Iine kinetic microplate muverengi uye endotoxin assay software, Endotoxin assay kit inoona endotoxin level muplasma yemunhu isingasviki awa imwe.Iyo kiti inouya ne plasma pre-treatment reagent iyo inobvisa iyo inhibition zvinhu muplasma panguva ye endotoxin assay.
2. Product Parameter
Chiyero chiyero: 0.01-10 EU/ml
3. Product Feature uye Application
Inouya neplasma pretreatment solutions, inobvisa inhibition zvinhu muplasma yemunhu.
Cherechedza:
Lyophilized Amebocyte Lysate (LAL) reagent inogadzirwa neBioendo inogadzirwa kubva kuamebocyte lysate inotorwa ropa rehoveshoe crab.
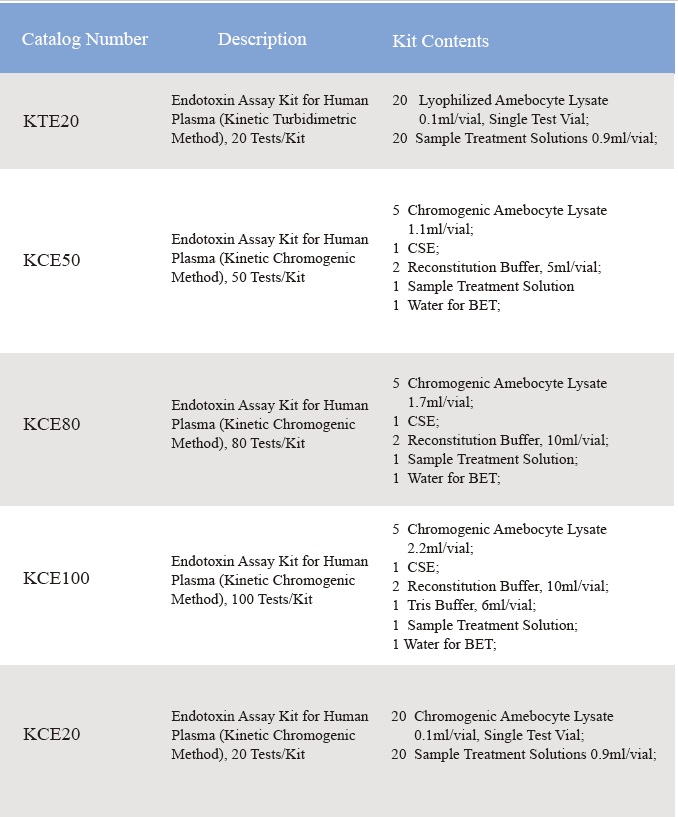
Kunzwa kweLyophilized Amebocyte Lysate uye potency yeKudzora Standard Endotoxin inoedzwa kurwisa USP Reference Standard Endotoxin.Iyo Lyophilized Amebocyte Lysate reagent kits inouya nemirairo yechigadzirwa, Chitupa cheAnalysis, MSDS.